Bakelite
Scandal & the Story of BakeliteBakelite hit the market in 1907, heralding the arrival of the modern plastics industry. Bakelite was the first completely man made plastic, as until then, plastics such as celluloid, casein, and Gutta-Percha all had as a base a natural material. It was developed by Belgian-born chemist Dr. Leo Hendrick Baekeland who started his firm General Bakelite Company to produce the phenolic resin type plastic. Bakelite was inexpensive to manufacture and extremely durable, and made its inventor a wealthy man. In subsequent generations, however, the Baekeland's family story was one of tragedy rather than triumph. In 1972 the schizophrenic great grandson of Dr. Baekeland stabbed his mother to death. Savage Grace, by Natalie Robbins and Steven M.L. Aronson, a book about the family and the murder, was a best seller when it was published in 1985.
Bakelite for Appliances & Jewelry
Early Bakelite was used almost exclusively in the manufacture of radios, appliances and electrical components because it was lightweight, inexpensive, durable, moisture-resistant and non-flammable. The limited color range of black, brown, and the occasional burgundy and dark green was appropriate for use as radio cabinets, vacuum cleaner parts, and electrical elements, but eventually, formulas were developed to produce the plastic in a range of appealing colors. Its ability to be carved and molded made it ideal for inexpensive jewelry. Early jewelry attempts to mimic more expensive materials like ivory amber, onyx, and jade, but by the 1930s, consumers began to appreciate the plastic for own qualities and Bakelite jewelry made its appearance everywhere from Sears Roebuck to Sacks 5th Avenue.
Colorful Art Deco Bakelite Gems
Artists and designers discovered the beauty and workability of Bakelite (and Catalin, a competitor who also produced a phenolic resin plastic). New technology created additional colors, and the plastic became available in scarlet, green, amber, brown burgundy, red-orange, and Kelly green and black and marbled. By 1934, yet another plastics company had produced a formula for Bakelite in pastel colors including willow green, light blue, pink and yellow. Due to the unstable nature of the chemicals used in the pastel colors formula, these pieces are hard to find, and as such, are among the most costly of Bakelite jewelry.
Bakelite Jewelry & Values
Bakelite could be molded, carved, or laminated, and designers and turned to the material for brightly colored, inexpensive flights of fancy to adorn everything from wrists to waists. Necklaces featured beads in a variety of sizes and colors, sometimes terminating in carved or laminated pendants. A popular choker style necklace consisted of pairs of bright red cherries on celluloid stems and leaves dangling from a celluloid chain (today $150-300.)
Bakelite Bracelets
Bracelets were stretchy, cuff, charm, wrap or tank-track styles. Stretchy bracelets consisted of beads or lozenges strung on elastic. Cuff styles could be wide and deeply carved, or narrower bands intended to be stacked together. The band could be smooth, molded (usually in a geometric pattern), carved, or pierced. Wrap bracelets were beads strung on wire, and tank-track bracelets featured overlapping semi-circular links. A quick check on eBay turned up bracelets on offer in prices ranging from $50-300. Deeply carved, wide red cuffs seem to fetch the highest prices, followed by amber, then green.
Bakelite Pins
A variety of pins were produced, either whimsical figurals or geometrics. The Art Deco love affair with the Scottie Dog was evident in the jewelry on offer. Horses also had a strong presence, but pins of elephants, penguins, marlins, and cherries are also available. Pins range in price from $118 for a lovely carved leaf, $130 for a carrot, $102 for a red horse head, and an almost shockingly low $18 for a classic Scottie in red.
Bakelite Jewelry Affordable (Again)!
Prices reached almost ludicrous levels in the early 1990s, and the jewelry became so popular that other Bakelite pieces such as poker chips, Tootsie Toys and Mah Jong tiles were frequently fashioned into jewelry. The market seems to have cooled, meaning that it's once again possible to buy a fine Bakelite cuff bracelet for less than a gold one.
Reference note by p4A Contributing Editor Susan Cramer, August, 2011
Bakelite
Bakelite is named after its inventor, Belgian-born chemist Leo Hendrik Baekeland (1863-1944). After emigrating to the United States in 1889, Baekeland dabbled in photography. In the late nineteenth century, photographic paper was so insensitive to light that prints had to be exposed outdoors in sunlight. Baekeland invented a more sensitive paper that he called Velox. He sold the rights to George Eastman in 1899 for a million dollars.Now independently wealthy, Baekeland bought a farm near Yonkers, New York and set up a laboratory in the barn. He wanted to develop an insulating coating for copper wire, the kind of wire used to wind solenoids and motors. In those days wire was coated with shellac, which was laboriously made from the shells of the lacca beetle that inhabited southeast Asia. Shellac was expensive and in short supply. Could Baekeland develop a synthetic substitute?
In 1907, he prepared a mixture of phenol, formaldehyde and lye which had the color and the consistency of honey. Unexpectedly, the mixture hardened in its container, producing a solid whose surface faithfully duplicated the shape and the texture of its container.
It occurred to Baekeland that his mixture could be heated in molds to create objects of any desired shape. Thus was born Bakelite, the world's first synthetic plastic. Baekeland founded The Bakelite Corporation to manufacture his material.
Bakelite is known by several generic names. It is referred to as phenolic because phenol (C6H5OH) is the main ingredient. Phenol is the preservative that is responsible for the "mediciney" smell of preschoolers' paste and is the "mediciney" ingredient in antiseptic mouthwash.
It is also known as thermosetting because the chemical reaction that creates the solid actually occurs while the molding compound is being heated in the mold. Once the solid object has been formed, it cannot be softened again, unlike thermoplastics such as polystyrene that can be melted and re-used. This property makes thermosets useful for objects that might become warm, such as housings for electrical devices or even handles for kitchen pots and pans.

A Manning-Bowman coffee pot with bakelite handle, base and spigot handle. (p4A item # D9810832)
Baekeland's competitors also made thermosets, and the word "bakelite" (small b) became a generic term denoting phenolic from any manufacturer. To further complicate things, The Bakelite Corporation later became a distributor of polystyrene, which was sold under the trade name Bakelite.
The original lump of Bakelite was a transparent amber-colored solid whose appearance Baekeland described as "frozen beer". A few products were actually molded that color, notably ladies' combs that were meant to simulate hand-carved tortoise shell.
Most Bakelite was made with additives that altered its appearance or mechanical properties.
Flock (short cotton threads) was often mixed with molding compound so that the threads would become embedded in the finished product. The fibers improved Bakelite's mechanical strength, much as steel reinforcing rods strengthen concrete.
The most common appearance for a Bakelite object was opaque black, which was produced by incorporating carbon black into the molding compound. The ubiquitous black rotary-dial telephone was manufactured of black Bakelite.

A Henry Dreyfuss bakelite telephone. (p4A item # B126428)
In the 1930s it became possible to make Bakelite in colors other than black by adding suitable pigments before molding. This led to the use of phenolic for costume jewelry and other decorative items.
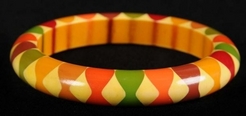
A bakelite "bow tie" pattern bangle bracelet. (p4A item # D9769092)
Bakelite objects are manufactured by a process known as compression molding. A pre-measured amount of molding compound is placed between two halves of a mold, which are then closed together. Initial heating softens the compound to the consistency of putty. High pressure forces the compound into every nook and cranny of the mold. Continued heating promotes the chemical reaction that produces the solid object. Automated molding presses could operate unattended, producing a finished object every 1-2 minutes.
Bakelite's popularity began to decline in the 1940s as thermoplastics became more readily available. The first major thermoplastic was cellulose acetate, which was made from a byproduct of the cotton gin. Intricately-shaped objects can be fabricated by injection molding. Molten plastic is forced into a mold under high pressure, where it cools and solidifies. Since cellulose acetate was derived from an agricultural product, supply could not keep pace with the growth of demand. After World War II, polystyrene (made from petroleum) quickly became the most popular thermoplastic.
The days of manufacturing collectible jewelry from Bakelite are over, but phenolics continue to be used for applications where heat resistance is required, such as electrical equipment or cookware.
Reference note by p4A Contributing Editor Joseph H. Lechner, Ph.D.